Products
Services
Applications
Resources
User Zone
Careers
Products
Peptide Synthesizers
Find a Product
View All Products
Services
Applications
Resources
About Us
Blog

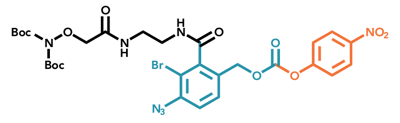
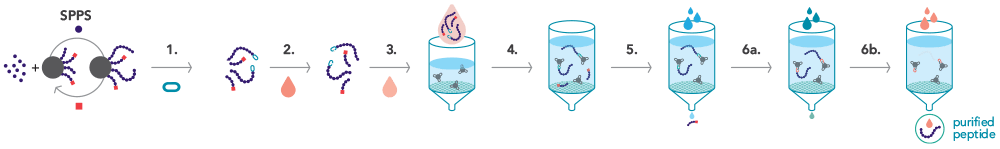
.png?width=400&height=300&name=Microscopy%20image%20of%20the%20activated%20agarose%20filter%20material%20(1).png)